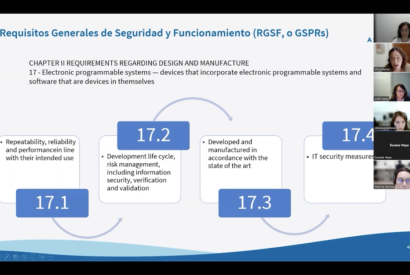
Tools for research and transfer in mental health in first person
On Wednesday 5th October at 9:30h, we will celebrate the 11th Breakfast&Learn of the Network, a session addressed to all scientists who want to include the first person in mental health research and transfer. Researcher Francisco Jose Eiroá will share tools and some keys to do it! Register!