Regulation of software as a medical device: risk class and process steps
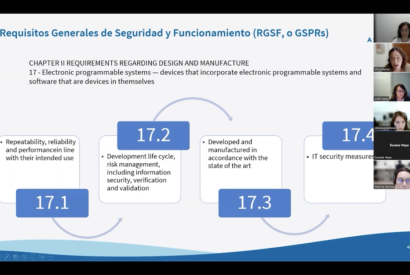
Available in VIDEO! Regulatory roadmap necessary to configure, validate and launch a software as a medical device (MD) in the 10th Breakfast&Learn of the TECSAM Network. Dominique Monferrer, Medical Device Director at Asphalion, showed us the classification of the software according to the risk class and the stages of the regulatory process. Don’t miss it!