
Research with gender perspective at the XVI B&L
Do you want to know ways-to-do, tools and success stories to include the gender perspective in research and transfer? Some keys at the B&L.

Do you want to know ways-to-do, tools and success stories to include the gender perspective in research and transfer? Some keys at the B&L.

Do you want to learn about mechanisms to boost the transfer of your research results? Biocat shares useful tools at the 15th B&L.

What is knowledge transfer? Learn about the step-by-step path to transferring solutions and the Network's support tools to make it possible.

More than 80 people attended the first face-to-face session of the TECSAM Network Kick-Off Meeting, a meeting point for member groups.

Do you want to learn about tools to transfer research results to the market? Discover how the TECSAM Network helps member groups in tech transfer.

Do you need funding to bring your solution to the market? At the 13th B&L, Patricio Hunt at Intelectium shares the 6 keys to attract private investors.
The TECSAM Network's new website is now available! Solutions portfolio, events, training capsules and more, just a click away. Dive into the world of innovation in mental health!

Looking for funding to develop and bring your research project to market? On 14 December we will be holding the 13th Breakfast&Learn, where we will share the steps to follow to access private funding. Conference by Intelectium. Sign up!
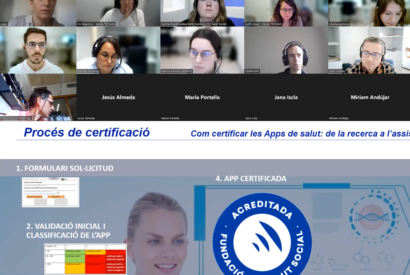
Certification of health apps: ethical principles, privacy and accessibility are some of the key aspects. This was one of the central reflections of the 12th B&L by TIC Salud Social, the official accreditation body in Catalonia. Now available in video!

On Wednesday 2 November at 9am, the TECSAM Network is holding the XII Breakfast&Learn, entitled “how to accredit or certify health Apps: from research to care” where Carme Pratpadua and Bufill from TIC Social Health will talk about the certification process, the roadmap and the main obstacles for the development of health apps. Join us!
IN VIDEO! Do you want to know the advantages and some tools for first-person research in mental health? In the XI Breakfast & Learn of the TECSAM Network, the researcher Francisco Jose Eiroá has given the keys to carry out participatory or co-produced research between researchers and people affected by mental problems. Did you miss the session or do you want to watch it again? Now available on video!

NEW LAUNCH! The TECSAM Network publishes the first Report on Mental Health Challenges Identified by Affected People, with the aim of including and involving affected people in the transfer of knowledge and value in the field of mental health. Download the document!