Certification of health apps: Ethics, privacy and accessibility, critical issues
Certification of health apps: ethical principles, privacy and accessibility are some of the key aspects. This was one of the central reflections of the 12th B&L by TIC Salud Social, the official accreditation body in Catalonia. Now available in video!
TIC Salud Social is the official Catalan body in charge of certifying that health apps meet the minimum criteria to be useful and reliable
Beyond the technical and functional criteria, researchers who develop health apps must comply with minimum ethical, data confidentiality and usability requirements in order to launch these technologies with full guarantees for users.
This was one of the reflections discussed by the expert Carme Pratdepàdua i Bufill, head of the mHealth area of the Fundació TIC Salut Catalunya, the official accreditation body for health apps in Catalonia, at the 12th Breakfast & Learn of the TECSAM Network. Video now available:
The problem facing the health app market is the lack of a specific regulation applicable to these technologies at European level – only those health apps considered medical devices are subject to the Medical Device Regulation – which means that there is no common regulatory framework and a single criterion and, therefore, each agency establishes its own parameters.
“We need a transversal regulation that allows us to cross borders”, explained Pratdepadua, as it is necessary to guarantee that the health applications that reach people have an objectifiable quality.
Even if an app has sufficient technological robustness, an elementary point for certification is that “the application follows ethical principles, with minimal intervention and asks for the minimum necessary data” from the user, said Pratdepàdua. She also mentioned as critical aspects the need to guarantee the privacy and security of users’ data and the importance of being an intuitive and inclusive technology.
Apart from CE certification, which is mandatory for the commercialization of all types of digital health technologies, TIC Salud Social is one of the 24 certifying agencies in the field of health apps throughout Europe, which aim to establish minimum quality, reliability and safety parameters for these products. The speaker also mentioned the emergence of a new ISO 82304-2 standard that could guarantee a certain standardisation of the sector at European level.
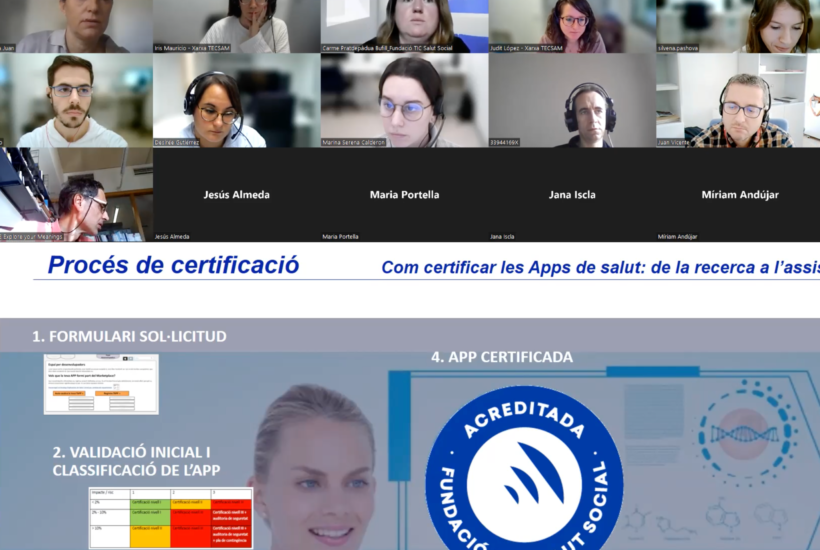
The TIC Salut certification process consists of 4 stages: a first application form where technical information is requested; the initial validation and classification of the app according to its potential impact on the population, the nature of the app (informative or data processing) and whether or not it gives recommendations to users; the rating according to the level of risk; and the last step is the certification of the app. This process takes approximately two months.
According to the speaker, the TIC Salut evaluation system is based on 120 mandatory, recommendable and desirable criteria. To be certified, apps must meet the minimum criteria necessary to be launched on the market, which varies depending on the app’s characteristics, purpose and intended use.
These criteria are grouped into 9 major blocks that are assessed by a multidisciplinary team of reviewers in charge of the resolution: ethics, privacy and security, reliable content and clinical part, development lifecycle, accessibility and usability, data sharing with system, development methodology, development language and environment, and app publication in the markets and sustainability.
Digital health is increasingly present in the medical sector, which makes it extremely important to have protocols and certifications to evaluate these new tools and guarantee the quality of what reaches the hands of society and users.
The video of the recorded session is now available. You can find more information here.

